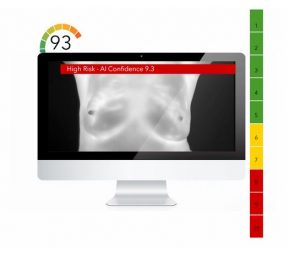
FDA: “There is no valid scientific data to show that thermographic devices, when used on their own or with another diagnostic test, are an effective screening tool for any medical condition, including the early detection of breast cancer or other diseases and conditions”.
The FDA is aware that health spas, homeopathic clinics, mobile health units, and other health care facilities are using thermography inappropriately as a standalone tool for breast cancer screening or diagnosis.
The FDA has received reports that these types of facilities provide false information that can mislead patients into believing that thermography is an alternative or better option than mammography. The FDA is concerned that people will believe the misleading claims about thermography. Read below to find out more about this topic.

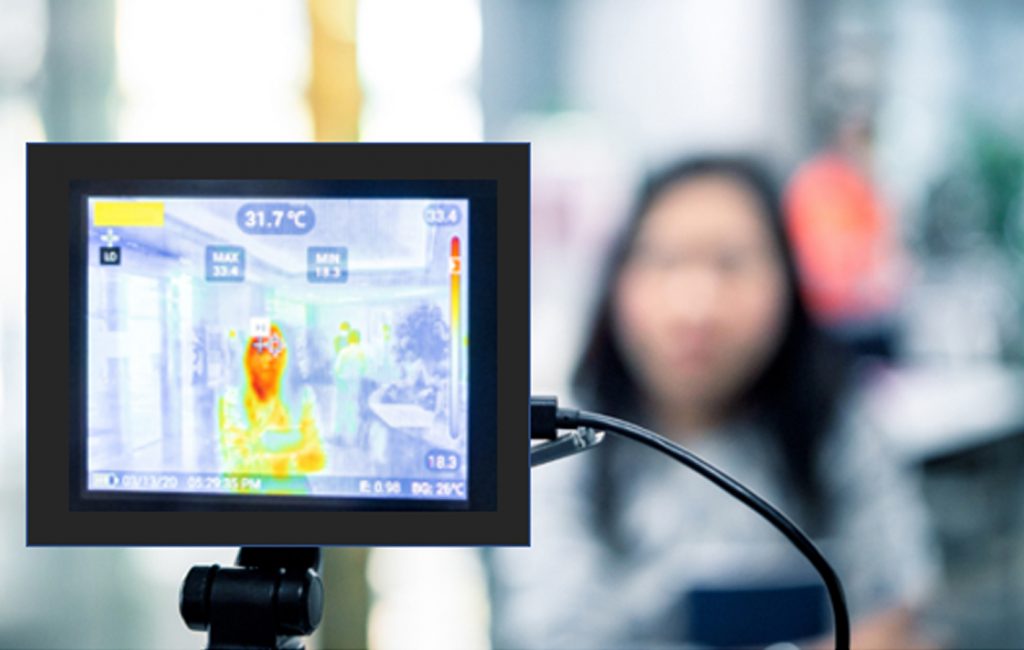
Since 1990s, several methods have been used for the early detection of breast cancer, such as mammography and MRI. Mammography is the standard method for early detection of breast cancer, but it has one main limitation: that is, it may produce a large number of false positives. In contrast, respective standard screening techniques are used for early detection of breast cancer such as Thermography. Thermal infrared images are used to detect lesions in breasts. Since the normal temperature range of human body is between 36.5∘C to 37.5∘C, by using this technique, thermal infrared cameras are able to capture the variation of temperature between normal and abnormal breast tissues. These Thermograms detect the temperature of the different regions of the breast. Regions with warmer temperature are more likely to contain tumors than a normal tissue. This technique has several substantial benefits:
- It is less expensive than the mammography and magnetic resonance imaging (MRI)
- It is non-contact, non-invasive
- It is non-radiative
- It is a safe diagnostic procedure, in which patients feel no pain
- This method can be used from far way
- It is possible to simultaneously monitor a large area of the population
- Interpretation of thermogram’s colors is easy and fast.
- This method only records natural radiation from the surface of the skin and there is no trace of harmful rays, so is suitable for long-term and repeating use.
- It is a fast way to monitor and observe the dynamic changes in temperature.
- Early detection up to 10 years before the cancer turns into a full-blown size
The tool, however, has been cleared by the FDA only as a supplement to primary diagnostic tests such as mammography and not as an substitute. Helen J. Barr, M.D., director of the Division of Mammography Quality Standards in the FDA’s Center for Devices and Radiological Health states that: “You should not rely solely on thermography for the screening or diagnosis of breast cancer.”
The agency affirmed that individuals who substitute thermography for mammography may miss the opportunity to detect cancer at its earliest and most treatable stages. The agency wrote in its safety communications statement that: “We are committed to protecting and promoting access to safe and effective breast screening devices.”
The United States Food and Drug Administration currently accept applications for thermographic cameras as Class I Medical Devices. These are described as devices that hold no potential risk and have the lowest level of regulatory control. Consequently, Experts highly recommend that the technique should only be used in connection with other breast cancer screening processes such as mammography. Moreover, it provides suggestions for individuals getting breast cancer screening to be aware of thermography as well as recommendations for health care providers to educate patients about the limitations of thermography.
Recommendations for People Getting Breast Cancer Screening
- Be aware that thermography is not a substitute for regular mammograms and should not be used in place of mammography for breast cancer screening or diagnosis.
- Have regular mammograms according to screening guidelines or as recommended by your health care provider.
- Follow your health care provider’s recommendations for additional steps to diagnose breast cancer such as a clinical breast exam, other breast imaging (for example, breast ultrasound or MRI), or breast tissue biopsy.
Recommendations for Health Care Providers
- Educate patients about the limitations of thermography. For example, the high false negative and false positive rates of thermography can provide misleading information that could result in a delayed diagnosis or unnecessary medical follow up.
- Discourage the use of thermography to diagnose or screen for breast cancer.
- Talk to patients or caregivers about safe and effective ways to screen for breast cancer including the benefits and risks of available testing options.
Reporting Problems to the FDA
If you experience an injury or adverse event with thermography, the FDA encourages you to file a voluntary report by phone at 1-800-FDA-1088 or online at MedWatch, the FDA Safety Information and Adverse Event Reporting program. Please include the following information in your reports:
- Device Name (Brand Name)
- Manufacturer’s Name
- Details of Adverse Event and Medical and/or Surgical Interventions (if applicable)
Prompt reporting of adverse events can help the FDA identify and better understand the risks related to the use of medical devices.
Additional Resources
- FDA Consumer Update: Breast Cancer Screening: Thermogram No Substitute for Mammogram
- FDA issues warning letter to clinic illegally marketing unapproved thermography device, warns consumers to avoid using thermography devices to detect breast cancer
- FDA Mammography Information
- FDA Consumer Update: Mammography: What You Need to Know
- Centers for Disease Control and Prevention (CDC) Breast Cancer Screening Information
- American College of Radiology – New Study Cements Fact That Mammography is a Primary Factor in Reduced Breast Cancer DeathsExternal Link Disclaimer
- National Cancer Institute – Breast Cancer Screening
- American Cancer Society Breast Cancer Screening Guideline
Contact Information
If you have questions about thermography, please contact www.aitalos.com or visit our linkedIn page: https://www.linkedin.com/company/ai-talos